
Aliphatic peptides show similar self-assembly to amyloid core sequences, challenging the importance of aromatic interactions in amyloidosis
A. Lakshmanan, D.W. Cheong, A. Accardo, E. Di Fabrizio, C. Riekel, and C.A.E. Hauser
PNAS, 110[2] (2013) 519-524
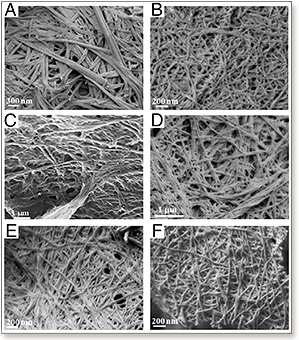
The self-assembly of abnormally folded proteins into amyloid fibrils is a hallmark of many debilitating diseases, from Alzheimer's and Parkinson diseases to prion-related disorders and diabetes type II. However, the fundamental mechanism of amyloid aggregation remains poorly understood. Core sequences of four to seven amino acids within natural amyloid proteins that form toxic fibrils have been used to study amyloidogenesis. We recently reported a class of systematically designed ultrasmall peptides that self-assemble in water into cross-beta-type fibers. Here we compare the self-assembly of these peptides with natural core sequences. These include core segments from Alzheimer's amyloid-beta, human amylin, and calcitonin. We analyzed the self-assembly process using circular dichroism, electron microscopy, X-ray diffraction, rheology, and molecular dynamics simulations. We found that the designed aliphatic peptides exhibited a similar self-assembly mechanism to several natural sequences, with formation of alpha-helical intermediates being a common feature. Interestingly, the self-assembly of a second core sequence from amyloid-beta, containing the diphenylalanine motif, was distinctly different from all other examined sequences. The diphenylalanine-containing sequence formed beta-sheet aggregates without going through the alpha-helical intermediate step, giving a unique fiber-diffraction pattern and simulation structure. Based on these results, we propose a simplified aliphatic model system to study amyloidosis. Our results provide vital insight into the nature of early intermediates formed and suggest that aromatic interactions are not as important in amyloid formation as previously postulated. This information is necessary for developing therapeutic drugs that inhibit and control amyloid formation.
DOI: 10.1073/pnas.1217742110

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
Al-Haytham Building (Bldg. 2)
© King Abdullah University of Science and Technology. All rights reserved