
Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents
N. Wiradharma, U. Khoe, C.A.E. Hauser, S.V. Seow, S. Zhang, and Y.Y. Yang
Biomaterials, 32 (2011) 2204-2212
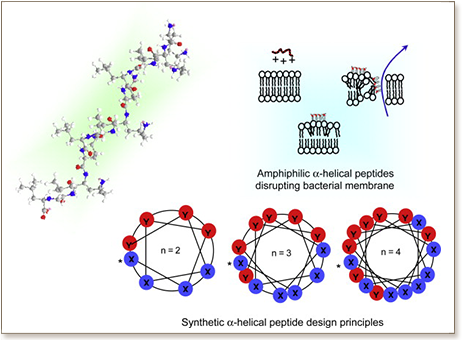
Antimicrobial peptides (AMPs) secreted by the innate immune system are prevalent as the effective first-line of defense to overcome recurring microbial invasions. They have been widely accepted as the blueprints for the development of new antimicrobial agents for the treatment of drug resistant infections. However, there is also a growing concern that AMPs with a sequence that is too close to the host organism's AMP may inevitably compromise its own natural defense. In this study, we design a series of synthetic (non-natural) short α-helical AMPs to expand the arsenal of the AMP families and to gain further insights on their antimicrobial activities. These cationic and amphiphilic peptides have a general sequence of (XXYY)n (X: hydrophobic residue, Y: cationic residue, and n: the number of repeat units), and are designed to mimic the folding behavior of the naturally-occurring α-helical AMPs. The synthetic α-helical AMPs with 3 repeat units, (FFRR)3, (LLRR)3, and (LLKK)3, are found to be more selective towards microbial cells than rat red blood cells, with minimum inhibitory concentration (MIC) values that are more than 10 times lower than their 50% hemolytic concentrations (HC50). They are effective against Gram-positive B. subtilis and yeast C. albicans; and the studies using scanning electron microscopy (SEM) have elucidated that these peptides possess membrane-lytic activities against microbial cells. Furthermore, non-specific immune stimulation assays of a typical peptide shows negligible IFN-α, IFN-γ, and TNF-α inductions in human peripheral blood mononuclear cells, which implies additional safety aspects of the peptide for both systemic and topical use. Therefore, the peptides designed in this study can be promising antimicrobial agents against the frequently-encountered Gram-positive bacteria- or yeast-induced infections.
DOI: 10.1016/j.biomaterials.2010.11.054

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
Al-Haytham Building (Bldg. 2)
© King Abdullah University of Science and Technology. All rights reserved