
The effect of thiol functional group incorporation into cationic helical peptides on antimicrobial activities and spectra
N. Wiradharma, M. Khan, L.-K. Yong, C.A.E. Hauser, S.V. Seow, S. Zhang, Y.-Y. Yang
Biomaterials, 32 (2011) 9100-9108
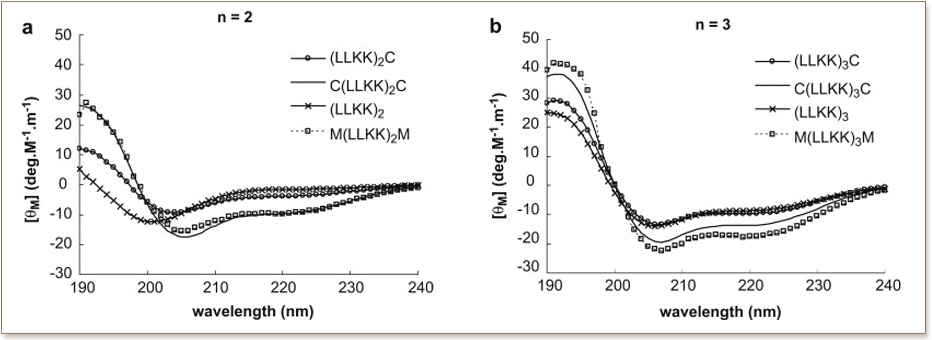
Antimicrobial peptides (AMP) have been proposed as blueprints for the development of new antimicrobial agents for the treatment of drug resistant infections. A series of synthetic AMPs capable of forming a-helical structures and containing free-sulfhydryl groups are designed in this study ((LLKK)(2)C, C(LLKK)(2)C, (LLKK)(3)C, C(LLKK)(3)C). In particular, the AMP with 2 cysteine residues at the terminal ends of the peptide and 2 repeat units of LLKK, i.e., C(LLKK)(2)C, has been demonstrated to have high selectivity towards a wide range of microbes from Gram-positive Bacillus subtilis, Gram-negative Escherichia coli, Pseudomonas aerogenosa, and yeast Candida albicans over red blood cells. At the MIC levels, this peptide does not induce significant hemolysis, and its MIC values occur at the concentration of more than 10 times of their corresponding 50% hemolysis concentrations (HC(50)). Microscopy studies suggest that this peptide kills microbial cells by inducing pores of similar to 20-30 nm in size in microbial membrane on a short time scale, which further develops to grossly damaged membrane envelope on a longer time scale. Multiple treatments of microbes with this peptide at sub MIC concentration do not induce resistance, even up to passage 10. However, the same treatment with conventional antibiotics penicillin G or ciprofloxacin easily develop resistance in the treated microbes. In addition, the peptides are shown not to induce secretion of IFN-gamma and TNF-alpha in human monocytes as compared to lipopolysaccharide, which implies additional safety aspects of the peptides to be used as both systemic and topical antimicrobial agents. Therefore, this study provides an excellent basis to develop promising antimicrobial agents that possess a broad range of antimicrobial activities with less susceptibility for development of drug resistance.
DOI: 10.1016/j.biomaterials.2011.08.020

"KAUST shall be a beacon for peace, hope and reconciliation, and shall serve the people of the Kingdom and the world."
King Abdullah bin Abdulaziz Al Saud, 1924 – 2015
Thuwal 23955-6900, Kingdom of Saudi Arabia
Al-Haytham Building (Bldg. 2)
© King Abdullah University of Science and Technology. All rights reserved